Products
Products
Ocean TuniCell AS has developed a tunicate nanocellulose product, TUNICELL, produced from a sustainable marine resource harvested on the west coast of Norway. We extract and process to > 99 % pure cellulose, free of contaminating hemicelluloses and lignin. This is then used to produce our ultrapure TUNICELL, with endotoxin values ≤ 0.5 EU/ml and bioburden < 10 CFU/ml. TUNICELL is produced according to ISO standards towards cGMP accreditation.
TUNICELL can be produced according to customer specifications at a range of concentrations up to 2.5 % nanocellulose in a dispersion. Our marine TUNICELL hydrogels are used in several research applications today, including wound healing, soft tissue reconstruction, cartilage repair, 3D cell culture and other bioengineering applications. See the publications below to learn how researchers are using TUNICELL for their applications today.
TUNICELL TTC
Tempo-mediated oxidized
Provided with or without an osmolyte (4.6% D-mannitol)

TUNICELL CTC
Carboxymethylated
Provided with or without an osmolyte (4.6% D-mannitol)

TUNICELL ETC
Enzymatically pretreated
Provided with or without an osmolyte (4.6% D-mannitol)

TUNICELL Dilution Kit
Cell culture grade water
Pyrogen free
Sterile accessories
Provided with or without an osmolyte (4.6% D-mannitol)

TUNICELL Starter Kit
TUNICELL Variants
Provided with or without an osmolyte (4.6% D-mannitol)
Publications
Apelgren P, Amoroso M, Säljö K, Montelius M, Lindahl A, Orrhult L, Gatenholm P, Kölby L (2021) Vascularization of tissue engineered cartilage – Sequential in vivo MRI display functional blood circulation, Biomaterials 276: 121002, https://doi.org/10.1016/j.biomaterials.2021.121002
Amoroso M, Apelgren P, Säljö K, Montelius M, Orrhult L, Engström M, Gatenholm P, Kölby L (2021) Functional and morphological studies of in vivo vascularization of 3D-bioprinted human fat grafts, Bioprinting 23: e00162, https://doi.org/10.1016/j.bprint.2021.e00162
Säljö K, Apelgren P, Orrhult L, Li S, Amoroso M, Gatenholm P, Kölby L (2022) Long-term in vivo survival of 3D-bioprinted human lipoaspirate-derived adipose tissue: proteomic signature and cellular content, Adipocyte: 11:1, 34-46, https://doi.org/10.1080/21623945.2021.2014179
Sämfors S, Niemi EM, Oskarsdotter K, Egea CV, Mark A, Scholz H, Gatenholm P (2022) Design and biofabricationof a leaf-inspired vascularized cell-delivery device, Bioprinting 26: e00199, https://doi.org/10.1016/j.bprint.2022.e00199
Kjesbu JS, Zaytseva-Zotova D, Sämfors S, Gatenholm P, Troedsson C, Thompson EM, Strand BL (2022) Alginate and tunicate nanocellulose composite microbeads – preparation, characterization and cell encapsulation. Carb Polymer 286: 119284, https://doi.org/10.1016/j.carbpol.2022.119284
Apelgren P, Sämfors S, Säljö K, Mölne J, Gatenholm P, Troedsson C, Thompson EM, Kölby L (2022) Biomaterial and biocompatibility evaluation of tunicate nanocellulose for tissue engineering, Bio Adv: 212828, https://doi.org/10.1016/j.bioadv.2022.212828
Gilljam K, Stenlund P, Standoft S, Andersen SB, Kaaber K, Lund H, MD, Bryn KRK (2023) Alginate and Nanocellulose Dressings With Extract From Salmon Roe Reduce Inflammation and Accelerate Healing of Porcine Burn Wounds, Burn Care & Research, 2023; irad006, https://doi.org/10.1093/jbcr/irad006
Boix-Lemonche G, Nagymihaly RM, Niemi EM, Josifovska N, Johansen S, Moe MC, Scholz H, Petrovski G (2023) Intra-Corneal Implantation of 3D Bioprinted Scaffolds Containing Mesenchymal Stromal Cells using Femtosecond-Laser-Assisted Intrastromal Keratoplasty. Macromol Biosci. 2:e2200422, https://doi.org/10.1002/mabi.202200422
Stenlund P, Enstedt L, Gilljam KM, Standoft S, Ahlinder A, Lundin Johnson M, Lund H, Millqvist Fureby A, Berglin M (2023) Development of an All-Marine 3D Printed Bioactive Hydrogel Dressing for Treatment of Hard-to-Heal Wounds. Polymers. 15(12):2627, https://doi.org/10.3390/polym15122627
Oskarsdotter K, Säljö K, Sämfors S, Niemi EM, Li S, Simonsson S, Apelgren P, Scholz H, Gatenholm P, Kölby L (2023) Autologous endothelialisation by the stromal vascular fraction on laminin-bioconjugated nanocellulose–alginate scaffolds, Biomed. Mat. 18:0405028, https://dx.doi.org/10.1088/1748-605X/acdebb
Oskarsdotter K, Nordgård CT, Apelgren P, Säljö K, Solbu AA, Eliasson E, Sämfors S, Sætrang HEM, Asdahl LC, Thompson EM, et al (2023) Injectable In Situ Crosslinking Hydrogel for Autologous Fat Grafting. Gels. 9(10):813. https://doi.org/10.3390/gels9100813
Xeroudaki M, Rafat M, Moustardas P, Mukwaya A, Tabe S, Bellisaro M, Peebo B, Lagali N (2023) A double-crosslinked nanocellulose-reinforced dexamethasone-loaded collagen hydrogel for corneal application and sustained anti-inflammatory activity. Acta Biomaterialia, 172, 234-248. https://doi.org/10.1016/j.actbio.2023.10.020
Product
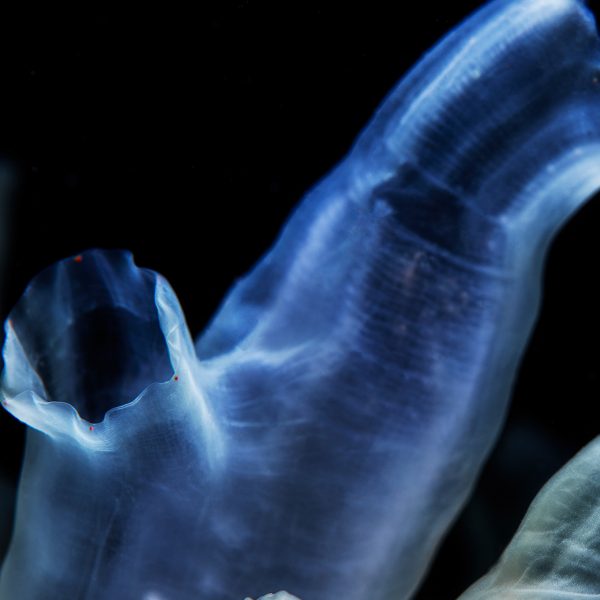
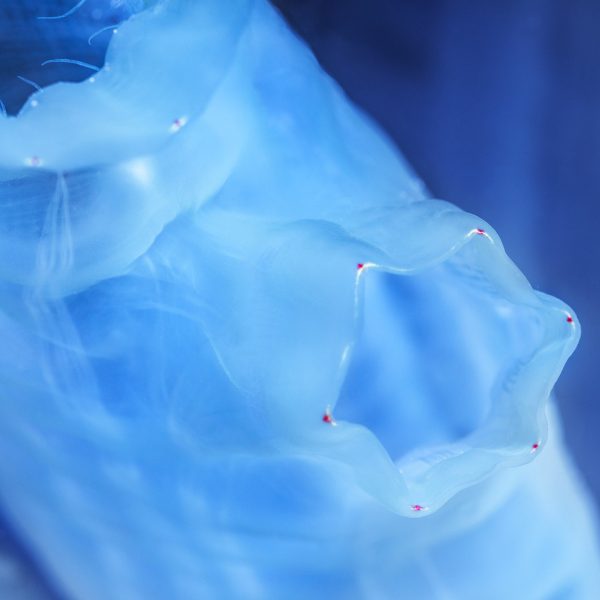
Our tunicate nanocellulose, TUNICELL, is produced from a sustainable, low-trophic marine resource, the tunicate Ciona intestinalis. Tunicates are the only animals that can synthesize cellulose, likely arising from the horizontal transfer of a prokaryotic cellulose synthase gene at the origin of the tunicate lineage.
Based on over 20 years of research, our team has developed the complete value chain from farming tunicates, to harvesting, processing, cellulose extraction and further refining of the tunicate cellulose to cellulose nanofibrils with tuneable properties.
The tunicates are harvested annually on the west coast of Norway, and during processing, different compartments are separated into components with individual commercial trajectories. Using our unique and specialized extraction protocol, one of these compartments, the animal “tunic”, yields tunicate cellulose that is of superior quality and purity, compared to cellulose isolated from plant-based sources. We further process this tunicate cellulose in our state-of-the-art cleanroom facilities to produce ultrapure medical grade cellulose nanofibrils, TUNICELL, according to ISO standards towards cGMP accreditation.
The physical characteristics and purity of TUNICELL make it an excellent scaffolding material in the emergent field of tissue and organ regeneration. Ocean TuniCell is currently supplying the market with an enzymatically pretreated medical grade TUNICELL product (TUNICELL ETC), TEMPO-mediated oxidized medical grade TUNICELL product (TUNICELL TTC), and carboxymethylated medical grade TUNICELL product (TUNICELL CTC). All products can be supplied with or without an osmolyte (4.6% D-Mannitol).
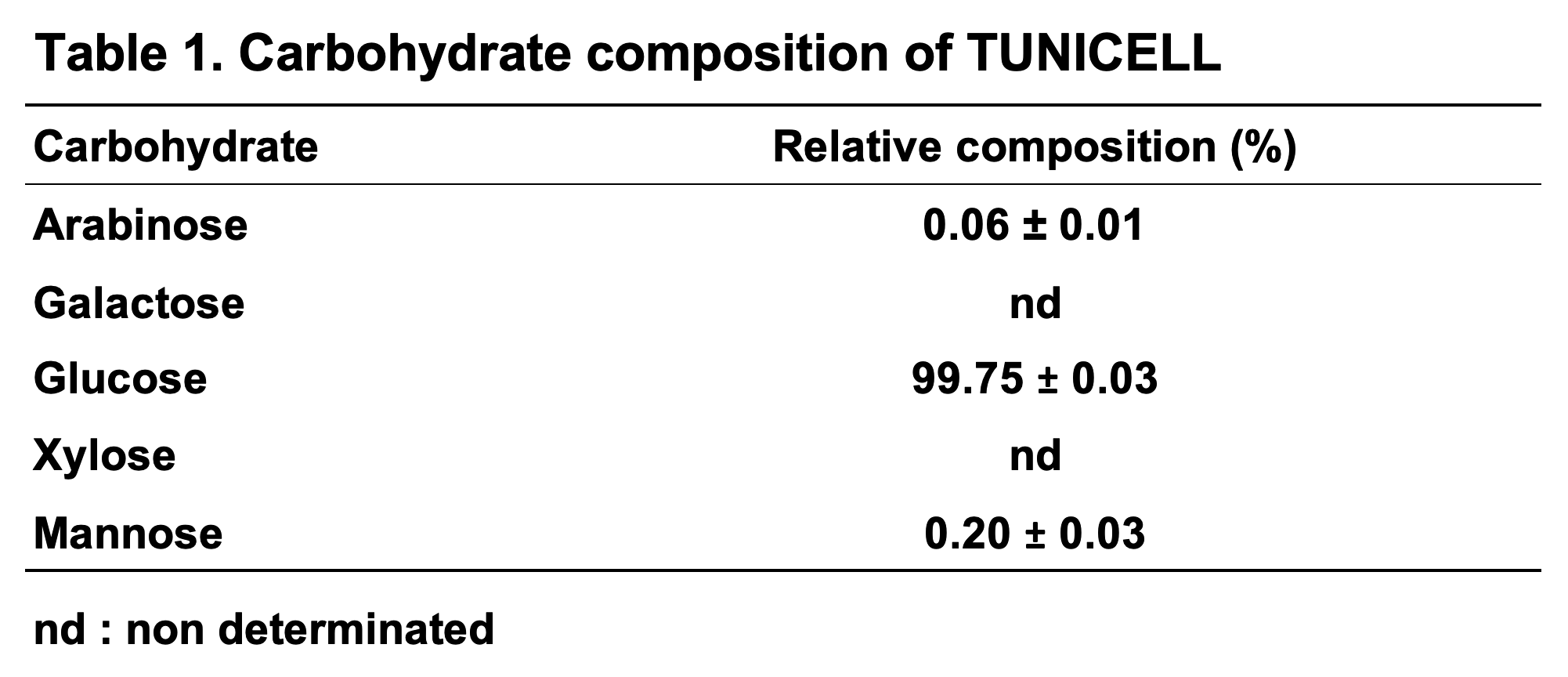
Tunicate cellulose has a purity of 99.75 ± 0.3 %, free of hemicellulose and lignin. The absence of heteropolymeric hemicellulose and lignin provides a highly controlled and reproducible scaffold material.
Carbohydrate composition of TUNICELL: Released carbohydrates after complete acid hydrolysis of TUNICELL were examined by high performance anion exchange chromatography with a pulsed amperometric detection (HPAEC-PAD) on a ICS3000 system (Dionex, Sunnyvale, CA, United States) using a Carbopac PA1 column (Dionex, Sunnyvale, CA, United States).
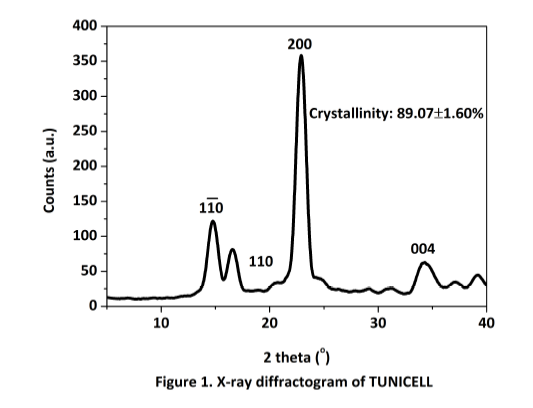
Tunicate cellulose is highly organized and our TUNICELL ETC has a crystallinity of 89.07 + 1.60 %, the highest found in nature. These physical characters yield a scaffold material with robust mechanical strength.
XRD: A PANalytical X’Pert PRO Materials research diffractometer equipped with an X’Celerator detector was used to determine the diffraction patterns and crystallinity index (CI) of TUNICELL. (Malvern Panalytical Ltd, UK).
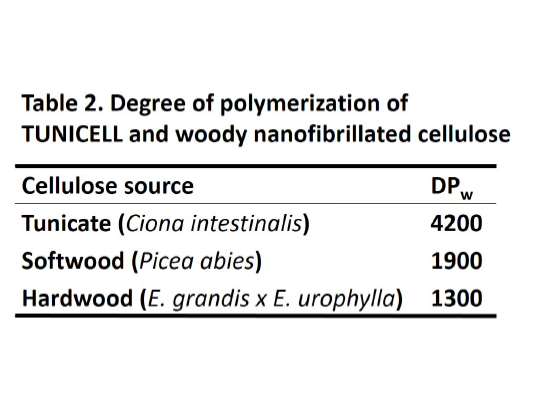
Tunicate cellulose fibers are much larger than traditional plant-derived cellulose fibers. This will contribute to greater stability of the scaffold. The data in Table 2 is based on analysis of TUNICELL ETC.
SEC: After complete dissolution of TUNICELL in LiCl/DMAc the molecular weight (or DPw) of TUNICELL was analyzed by a size exclusion chromatography (SEC) system equipped with a Rheodyne injector, a DGU-20A3 degasser, a LC-20AD liquid chromatography system, and a RID-10A refractive index detector (Shimadzu, Kyoto, Japan).
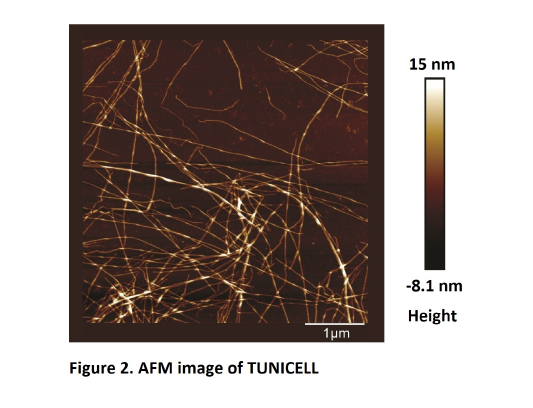
Tunicate cellulose (TUNICELL ETC) has long (1598 ± 822 nm) and thick (4.49 ± 1.58 nm) fibers with a high aspect ratio (382 ± 150) yielding a large reactive surface. This will favor modifications and cross linking with other materials in devices and other applications.
AFM: A tapping-mode atomic force microscope (AFM, Multimode IIIa, Veeco, Santa Barbara, CA, United States) was used to determine the morphology of TUNICELL.

Our medical grade ultrapure nanocellulose is produced under clean room standards, is sterilized and has bioburden levels of < 10 CFU/ml and endotoxin levels ≤ 0.5 EU/ml, adhering to FDA regulations for medical devices.
Endotoxin: Levels are tested by Lonza’s PyroGene™ Recombinant Factor C Assay. Bioburden: The bioburden test is conducted according to European Pharmacopoeia, Chapter 2.6.12.

The first medical grade tunicate nanocellulose on the market! Tunicate cellulose has a purity of 99.2 ± 0.3 %, free of hemicellulose and lignin. The absence of heteropolymeric hemicellulose and lignin provides a highly controlled and reproducible scaffold material.
Carbohydrate composition of TUNICELL: Released carbohydrates after complete acid hydrolysis of TUNICELL were examined by high performance anion exchange chromatography with a pulsed amperometric detection (HPAEC-PAD) on a ICS3000 system (Dionex, Sunnyvale, CA, United States) using a Carbopac PA1 column (Dionex, Sunnyvale, CA, United States).

Tunicate cellulose is highly organized and our TUNICELL ETC has a crystallinity of 89.07 + 1.60 %, the highest found in nature. These physical characters yield a scaffold material with robust mechanical strength.
XRD: A PANalytical X’Pert PRO Materials research diffractometer equipped with an X’Celerator detector was used to determine the diffraction patterns and crystallinity index (CI) of TUNICELL. (Malvern Panalytical Ltd, UK).

Tunicate cellulose fibers are much larger than traditional plant-derived cellulose fibers. This will contribute to greater stability of the scaffold. The data in Table 2 is based on analysis of TUNICELL ETC.
SEC: After complete dissolution of TUNICELL in LiCl/DMAc the molecular weight (or DPw) of TUNICELL was analyzed by a size exclusion chromatography (SEC) system equipped with a Rheodyne injector, a DGU-20A3 degasser, a LC-20AD liquid chromatography system, and a RID-10A refractive index detector (Shimadzu, Kyoto, Japan).

Tunicate cellulose (TUNICELL ETC) has long (1598 ± 822 nm) and thick (4.49 ± 1.58 nm) fibers with a high aspect ratio (382 ± 150) yielding a large reactive surface. This will favor modifications and cross linking with other materials in devices and other applications.
AFM: A tapping-mode atomic force microscope (AFM, Multimode IIIa, Veeco, Santa Barbara, CA, United States) was used to determine the morphology of TUNICELL.

Our medical grade ultrapure nanocellulose is produced under clean room standards, is sterilized and has bioburden levels of < 10 CFU/ml and endotoxin levels ≤ 0.5 EU/ml, adhering to FDA regulations for medical devices.
Endotoxin: Levels are tested by Lonza’s PyroGene™ Recombinant Factor C Assay. Bioburden: The bioburden test is conducted according to European Pharmacopoeia, Chapter 2.6.12.

The Technology
TUNICELL is a unique material made to facilitate the translational process in tissue engineering and regenerative medicine.
Sustainably and responsibly produced in line with the UN’s Sustainable Development Goals, with superior quality, and reproducibility – always to medical grade standards.
Sustainable Production
In the fjords of Bergen, Norway we cultivate one species of marine filter feeder – Ciona Intestinalis.
This organism, and other tunicates, are unique as they are the only known animal group to produce cellulose. The cellulose from C. intestinalis resembles our body’s own collagen in structure, but is has a high mechanical strength and it is an ultrapure polymer consisting of over 99% glucose, which is familiar to our bodies and cells.
These properties makes TUNICELL very well suited for biomedical applications such as tissue engineering and regenerative medicine. The possibilities for modifications allows researchers to create relevant in vitro models where they have complete control over matrix composition and factors that may influence data and results.