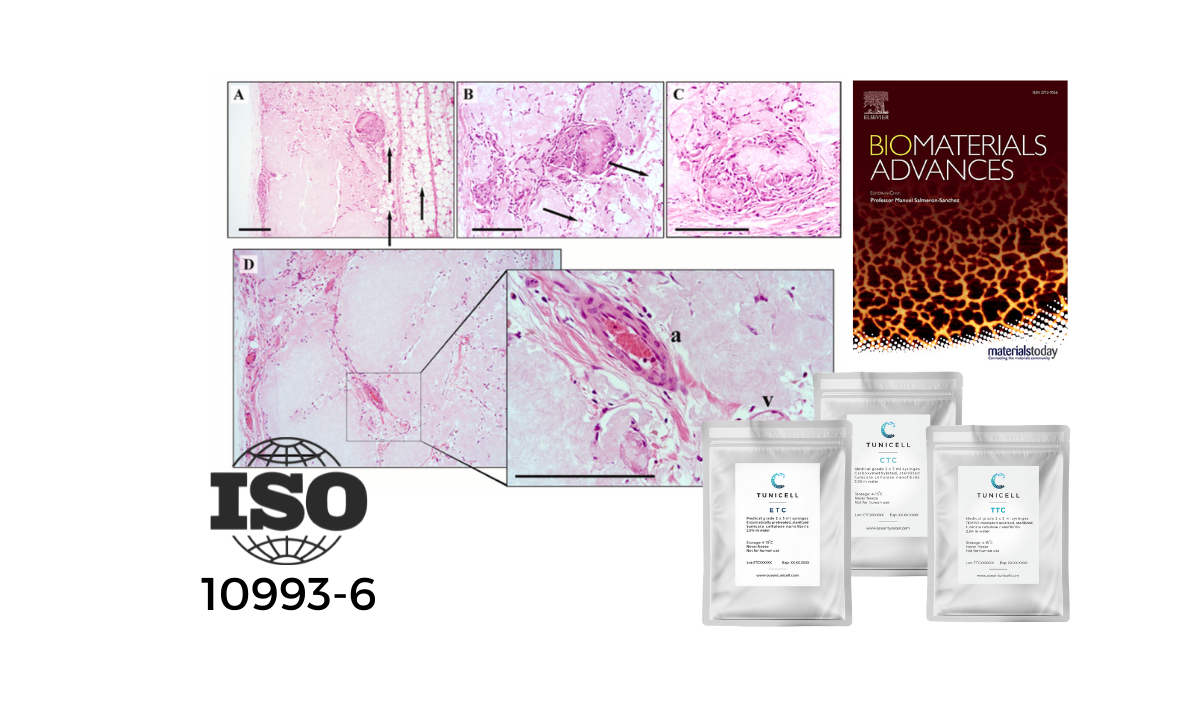
In a recent study by Apelgren et al. 2022 published in Biomaterials Advances, TUNICELL was shown to be biocompatible according to ISO 10993-6:2016, which is a biological evaluation of medical devices, testing for local effects after implantation.

“This is a milestone for Ocean TuniCell. TUNICELL combined with living cells could become the next gold standard for organ and tissue engineering”, says Christofer Troedsson, who is co-founder and CEO at Ocean TuniCell. He adds “The ultrapurity combined with mechanical strength of TUNICELL allows us to engineer clinically relevant tissues with high structural integrity.”
TUNICELL fibrils present similar dimensions to collagen, are chemically well defined and have a cost-efficient industrialized process to medical grade standards. In this latest study by Apelgren and colleagues, the physical properties of TUNICELL were characterized and its biocompatibility was assessed compared to a surgical polytetrafluoroethylene (ePFTE) mesh as a control.
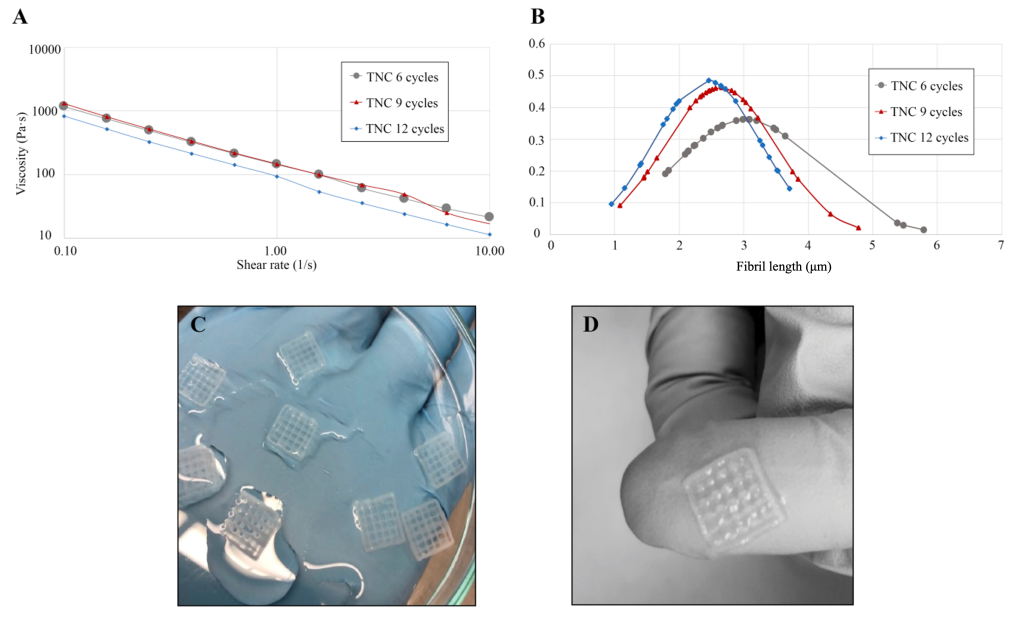
The average length of TUNICELL fibrils can be tuned to adjust mechanical strength for different applications. TUNICELL has high shear-thinning properties in rheological analyses, with adjustable viscosity dependent on fibril lengths and concentration. TUNICELL constructs can be printed with high resolution and fidelity.
In this study, TUNICELL was implanted in Wistar rats. After 30 days in vivo, the structural integrity of constructs was preserved. After 90 days the constructs remained structurally intact, and showed no signs of necrosis, infection, acute inflammation, invasion of neutrophil granulocytes, or extensive fibrosis.

“Tunicate nanocellulose fibrils (TUNICELL) have similar dimensions to the major extracellular matrix protein, collagen, but can be tuned over a much larger range of mechanical strengths. The large reactive surface area of the fibrils also permits grafting of a diversity of specific biological signals. The combination of these features make ultrapure TUNICELL an excellent scaffolding material for a wide range of applications in tissue engineering and 3D cell culture.” says Dr. Eric Thompson, who is co-founder and CSO at Ocean TuniCell.
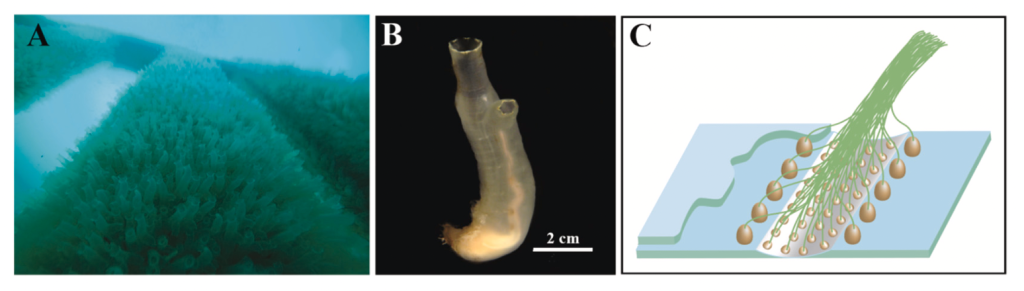
Tunicate aquaculture was started many years ago on the west coast of Norway by a group of scientists from the University of Bergen and NORCE. Together with their colleagues, this group founded Ocean TuniCell AS to build a new biotech industry in Bergen. You can read more of their story here:
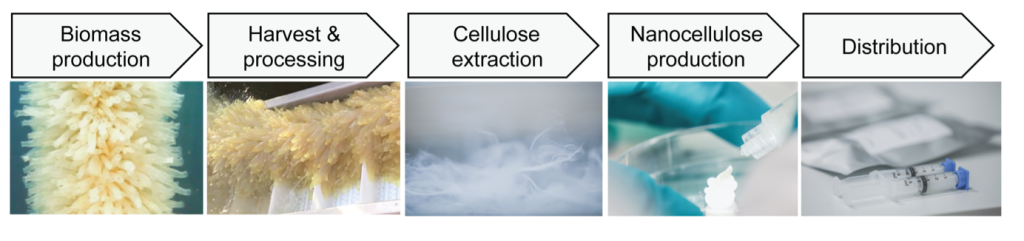
Medical grade TUNICELL is produced in GMP compliant clean room facilities, and used globally by medical researchers and biotech businesses. The company now has 13 employees working throughout the value chain from aquaculture to harvesting, cellulose refining, and nanocellulose production. At Ocean TuniCell we combine decades of experience in tunicate molecular biology, biopolymer chemistry, marine engineering and business entrepreneurship, dedicated to providing superior quality nanocellulose for scaffolding in biomedical applications.
The results in the publication by Apelgren et al. (2022) that TUNICELL is considered biocompatible according to the ISO standard 10993-6, is a major milestone for moving this venture forward.
P. Apelgren, S. Sämfors, K. Säljö, J. Mölne, P. Gatenholm, C. Troedsson, E. Thompson, L. Kölby, (2022). Biomaterial and biocompatibility evaluation of tunicate nanocellulose for tissue engineering. Biomaterials Advances, 212828. https://doi.org/10.1016/j.bioadv.2022.212828